NATIONWIDE — Federal drug officials have issued a recall for hundreds of thousands of a brand of insulin pump that they say injured thousands and caused one death.
- Over 322,000 units being recalled
- Problem with retainer ring affecting insuling dosage
- Company: 2,175 reports of injuries, 1 death
- RELATED: Find More Product Recalls
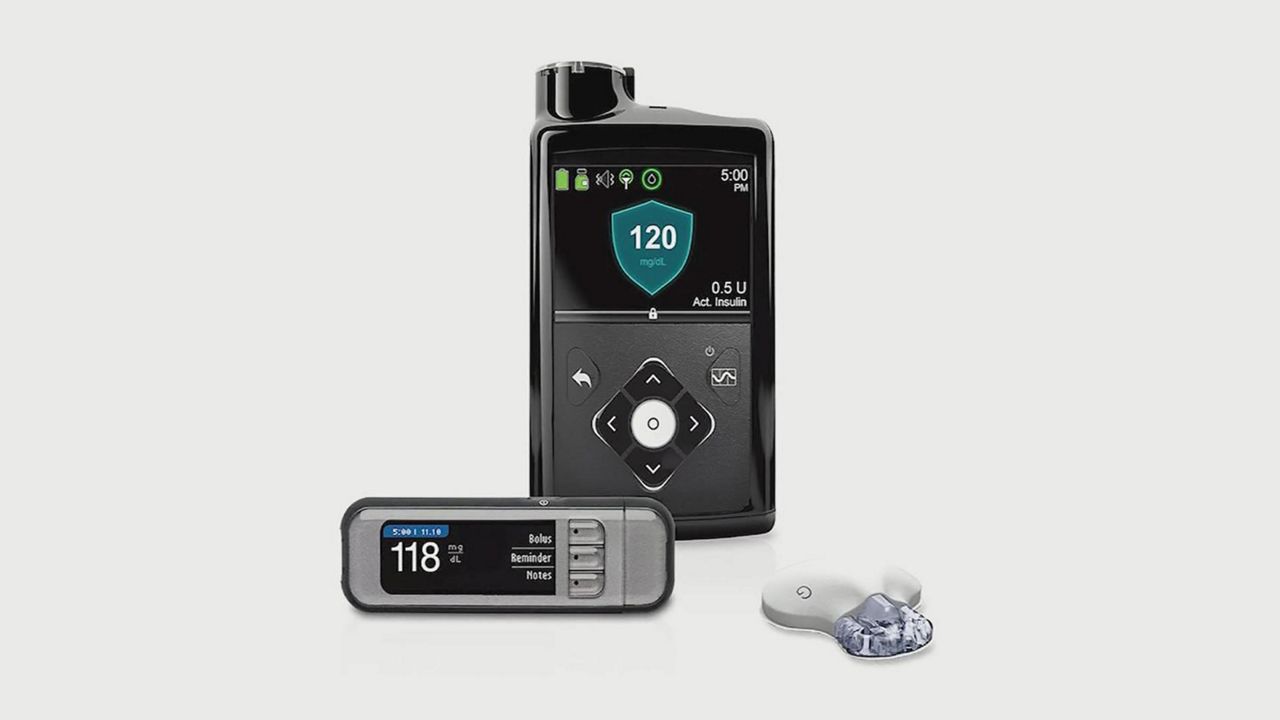
The recall is for the MiniMed Insulin Pumps models 630G and 670G, manufactured by Medtronic and used by people with Type 1 diabetes.
Medtronic says the pumps have a missing or broken retainer ring, which helps keep the insulin cartridge in place. If the cartridge is not firmy locked in, the units can deliver too much or too little insulin to the patient.
The company says it has received 26,421 complaints about the device malfunctioning, including 2,175 injuries and one death.
In November, the company told customers about the issue, but on Wednesday, the Food and Drug Administration issued a Class I recall, which is considered the most serious type of recall.
The company is recalling 322,005 units, distributed between September 2016 and October 2019.
Customers should stop using the device and call Medtronic for a replacement pump if you notice problems getting the pump to lock into place, or if the retainer ring is loose, damaged, or missing. If you have any questions, call Medtronic's 25-hour technical support line at 877-585-0166.